Demonstrate why in compound A,the nitrogen is more likely to act as a base,whereas in compound B,the oxygen atom is more likely to act as a base.Use resonance structures to justify your answers. 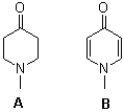
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q68: Indicate which atoms are part of the
Q69: The following molecule shown below is Lipitor,a
Q70: A molecule that is aromatic has _
Q71: Draw two other resonance states of naphthalene
Q72: Benzyne (shown below)is a highly reactive yet
Q74: Conjugated systems tend to add rigidity to
Q75: Which molecule shown below would you expect
Q76: HOMO is an acronym for the _
Q77: Show electron flow and draw a resonance
Q78: A molecule that follows all criteria for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents