Which resonance is more heavily favoured and why? 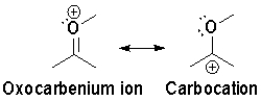
A) the oxocarbenium ion,because ð bonds are more stable than lone pairs
B) the oxocarbenium ion,because all atoms have a full octet
C) the carbocation,because the positive charge is on the least electronegative atom
D) the carbocation,because lone pair electrons are more stable than ð bonds
Correct Answer:
Verified
Q7: What is the electrophilic reactant B in
Q8: Which of the following best describes a
Q9: Which resonance state contributes most to the
Q10: What is the role of ethanol in
Q11: Which of the following best describes the
Q13: Which functional group is more reactive as
Q14: Which of the following best describes the
Q15: In the following Grignard reaction,what are the
Q16: Why is the oxocarbenium ion the more
Q17: What is the product of the following
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents