Consider the following equilibrium of a ketone hydration shown below.Which reactant would have the largest equilibrium constant for this reaction? 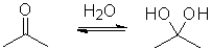
A) 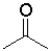
B) 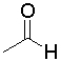
C) 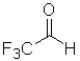
D) 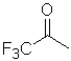
Correct Answer:
Verified
Q30: Which of the following best describes the
Q31: Which of the following reactants would most
Q32: Which of the following best describes the
Q33: Which reagent best reduces an imine to
Q34: What would be the most likely product
Q36: What is the expected major product of
Q37: What is the functional group shown below?
Q38: Considering the cyanide ion CN-,which atom acts
Q39: What are the partial charges at each
Q40: Which of the following molecules will undergo
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents