What is the role of acid in the following reaction? 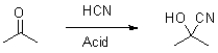
A) to protonate the negatively charged cyanide
B) to stabilize the ketone
C) to protonate the formed alkoxide
D) to form hydrogen bonds with the nitrogen in the cyanide ion
Correct Answer:
Verified
Q22: Which of the following molecules will undergo
Q23: What is the expected major product of
Q24: What is a major difference between acid
Q25: Which orbital of a carbonyl group accepts
Q26: What would be the most likely product
Q28: Which molecule would undergo the fastest reduction
Q29: What is the functional group shown below?
Q30: Which of the following best describes the
Q31: Which of the following reactants would most
Q32: Which of the following best describes the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents