Which proton in the following molecule is more acidic and why? 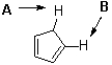
A) A,because of charge distribution
B) A,because of hybridization
C) B,because of charge distribution
D) B,because of hybridization
Correct Answer:
Verified
Q28: A Lewis acid is an electron acceptor.
Q29: Acidity increases going down the periodic table.
Q30: Compound A has a pKa of 10
Q31: Rank the labelled protons in the following
Q32: Which proton is expected to be the
Q34: Which of the indicated protons in the
Q35: Which of the following best describes the
Q36: A Brønsted base is a molecule that
Q37: Rank the labelled amines in the following
Q38: Given the following reaction scheme,which molecule is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents