The hydrogen atoms on the sp3-hybridized carbon are much more acidic then their sp2-hybridized counterparts in 1,3-cyclopentandiene,seemingly violating the rule that atoms with more s character are more acidic.Explain why this is the case.Draw a picture of the molecule to justify your answer. 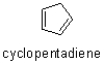
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q70: Tert-butanol is 100 times less acidic then
Q71: A chemist mixes propanoic acid with one
Q72: Predict the products of the following acid/base
Q73: When considering atomic size,_ atoms are better
Q74: Would water be a suitable solvent for
Q75: Alkyl groups are considered inductively electron _
Q76: Although similar constitutional isomers,salicylic acid is 50
Q77: Use resonance structures to explain the differences
Q78: Oxygen is better able to stabilize negative
Q80: The stronger the acid,the _ the conjugate
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents