Substituents on phenol can modulate its pKa values.Explain the differences in pKa seen in the three different phenol molecules shown below.Draw resonance structures to justify your answers. 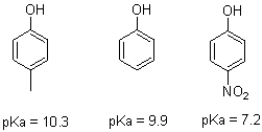
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q61: A carbanion is an example of a
Q62: Neighbouring _ groups stabilize a conjugate base
Q63: Electronegative atoms are _ capable of carrying
Q64: Given the following reaction equilibrium and pKa
Q66: Sulfur is better at stabilizing negative charge
Q67: A molecule with an empty p orbital
Q68: Determine which side of the equilibrium is
Q69: A molecule will be deprotonated If the
Q70: Tert-butanol is 100 times less acidic then
Q79: A carbocation is an example of a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents