Figure 1
Shown below is the structure of caffeine in the centre and two different resonance states I and II with lone pairs and formal charges omitted. 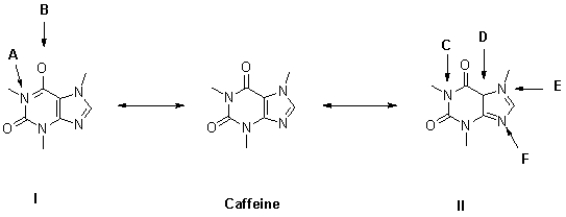
-Referring to Figure 1,what is the formal charge of the oxygen atom at B?
A) +1
B) 0
C) -1
D) -2
Correct Answer:
Verified
Q1: Which of the following has the biggest
Q2: What do curved arrows in organic reaction
Q4: What is the formal charge of the
Q5: Which of the following best describes carbanions?
A)They
Q6: Which of the following best describes an
Q7: Figure 1
Shown below is the structure of
Q8: how many lone pair electrons are around
Q9: Which of the following arrows represents resonance
Q10: Which of the following is NOT a
Q11: What is the formal charge around the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents