Given the following Lewis structure,how many lone pairs are expected to be around the sulfur and oxygen atom? 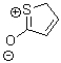
A) sulfur = 2,oxygen = 2
B) sulfur = 1,oxygen = 2
C) sulfur = 2 oxygen = 3
D) sulfur = 1 oxygen = 3
Correct Answer:
Verified
Q27: Both ð and ó bonds can be
Q28: Figure 1
Shown below is the structure of
Q29: What is the product or products of
Q30: Curved arrows in reaction mechanism show the
Q31: Figure 1
Shown below is the structure of
Q33: Figure 1
Shown below is the structure of
Q34: Figure 1
Shown below is the structure of
Q35: Which of the following is the most
Q36: Figure 1
Shown below is the structure of
Q37: Figure 1
Shown below is the structure of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents