Which of the following molecules would be expected to have the largest melting point?
A) 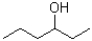
B) 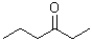
C) 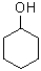
D) 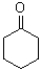
Correct Answer:
Verified
Q47: What is the IUPAC name of the
Q48: A nitrile bond contains two ð bonds.
Q49: The stronger the intermolecular forces,the higher the
Q50: Aldehydes have a suffix of -al when
Q51: Diethyl ether is a non-polar solvent.
Q53: What is the IUPAC name of the
Q54: But- is a prefix that represents three
Q55: Branched hydrocarbons are capable of more London
Q56: Branched hydrocarbons have higher boiling points than
Q57: Substituents on an organic molecule are named
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents