Which of the labelled carbons in the molecule shown below is the most electron rich and which is the most electron deficient? 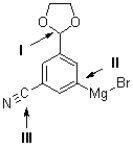
A) I is the most electron rich;II is the most electron deficient.
B) II is the most electron rich;I is the most electron deficient.
C) I is the most electron rich;III is the most electron deficient.
D) III is the most electron rich;I is the most electron deficient.
Correct Answer:
Verified
Q54: Only filled molecular orbitals contribute to bonding.
Q55: Anti-bonding orbitals are lower in energy than
Q56: Resonance structures contain delocalized electrons.
Q57: An sp3 hybridized atom has a tetrahedral
Q58: An sp2 hybridized atom has a trigonal
Q60: The resonance hybrid is the most stable
Q61: Overlap of p orbitals is known as
Q62: The two ð bonds in an triple
Q63: A triple bond contains three ð bonds.
Q64: An sp hybridized carbon has an orbital
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents