Assign non-zero formal charges to the following molecule. 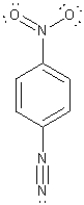
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q63: A triple bond contains three ð bonds.
Q64: An sp hybridized carbon has an orbital
Q65: Hybridized orbitals are capable of resonance.
Q66: Empty p orbitals are incapable of contributing
Q67: Triple bonds are not capable of contributing
Q69: Anti-bonding orbitals involve out of plane overlap
Q70: A Nitrogen atom with four ó bonds
Q71: Assign non-zero formal charges and the hybridization
Q72: A carbocation with three ó bonds is
Q73: Only carbon atoms can hybridize.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents