Which set of conditions will accomplish the following transformation? 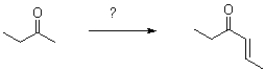
A) 1) 1.05 eq.LDA,inverse addition -78°C 2) Ethanal 3) H3O+/heat
B) 1) 0.98 eq.LDA -78°C to 0°C 2) Ethanal 3) H3O+/heat
C) 1) 1 eq.NaOMe -78°C 2) Ethanal 3) H3O+/heat
D) 1) 1 eq.NaOMe 20°C 2) Ethanal 3) H3O+/heat
Correct Answer:
Verified
Q18: Which of the following conditions best promotes
Q19: What is the product of an á,â-unsaturated
Q20: Which of the following best describes the
Q21: What is the product of the transformation
Q22: What is the product of the reaction
Q24: Which of the following is NOT a
Q25: What would be the major product of
Q26: Which of the following represents the kinetic
Q27: Which of the following represents the thermodynamic
Q28: Which of the following is the major
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents