The following reaction results in two enantiomers,with one of the enantiomers existing in excess.Predict which enantiomer will be the major product,and show an intermediate that influences this result. 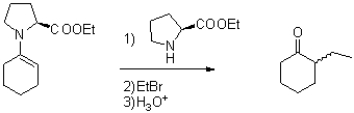
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q60: A cuprate is an example of an
Q61: Predict the structure of the product shown
Q62: 1,4-addition to an á,â-unsaturated carbonyl is also
Q63: A Z-enolate will form a(n)_ aldol product
Q64: Molecules with oxy substituents separated by an
Q66: The kinetic enolate has the _ substituted
Q67: Draw a mechanism of the following transformation.
Q68: The alternating reactivity pattern of consecutive carbons
Q69: Stabilized ylides contain a neighbouring _ group.
Q70: Draw the mechanism of the following transformation.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents