Which cyclohexane derivative would you expect to react faster under E2 elimination conditions and why? 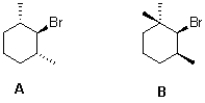
A) A because its less sterically hindered
B) B because its less sterically hindered
C) A because its scissile C-H bond is antiperiplanar
D) B because its scissile C-H bond is antiperiplanar
Correct Answer:
Verified
Q17: What is the first step in an
Q18: Which of the following represents the first
Q19: Which of the following reaction mechanisms would
Q20: For an E2 elimination of bromocyclohexane to
Q21: Which of the following best describes sodium
Q23: Which of the following reaction mechanisms would
Q24: Which of the following reaction mechanisms would
Q25: What would be the major product of
Q26: Which of the following mechanisms are promoted
Q27: Which of the following best describes the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents