What is the role of sulfuric acid in the following reaction scheme? 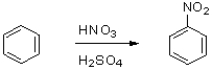
A) to protonate the aromatic ring and make it a better electrophile
B) to form dipole interactions with the developing positive charge in the aromatic ring
C) to protonate nitric acid to form the nitronium ion
D) to regenerate nitric acid
Correct Answer:
Verified
Q1: Why is nitrobenzene NOT compatible with Friedel-Crafts
Q2: What set of reagents would most effectively
Q3: What is the role of FeBr3 in
Q4: As substituents on aromatic rings,which of the
Q5: Which of the following would be the
Q7: Which of the following would be the
Q8: What route would work best for the
Q9: What would be the expected product of
Q10: Why is aniline NOT compatible with Friedel-Crafts
Q11: Which of the following is an intermediate
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents