Which molecule will react faster with Br2 in the presence of iron bromide and why? 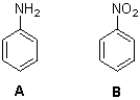
A) A because the amino group makes the ring more negative through charge delocalization
B) B because the nitro group makes the ring more negative through charge delocalization
C) A because the amino group makes the ring less negative through charge delocalization
D) B because the nitro group makes the ring less negative through charge delocalization
Correct Answer:
Verified
Q31: Which of the following compounds will the
Q32: Which of the following compounds will the
Q33: What is the common name for methoxybenzene?
A)analine
B)anisole
C)phenol
D)toluene
Q34: Which of the following statements best describes
Q35: Which of the following molecules would be
Q37: In an SEAr reaction,how would you describe
Q38: Which of the following best describes a
Q39: Which of the following compounds will the
Q40: In an SEAr reaction,how would you describe
Q41: Carbocation rearrangements can involve either the movements
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents