A compound with molecular formula C6H15N exhibits a singlet at d 0.9 (1H) ,a triplet at d 1.10 (3H) ,a singlet at d1.15 (9H) ,and a quartet at d 2.6 (2H) in its 1HNMR spectrum.Its IR spectrum shows one medium absorption band near 3400 cm-1.What is the structure of this compound? 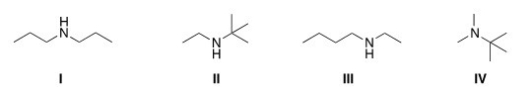
A) I
B) II
C) III
D) IV
Correct Answer:
Verified
Q31: Predict the product(s)of the following reaction.
Q32: Why are alkylamines more basic than arylamines?
A)The
Q33: In the preparation of primary amines,how can
Q34: Why is the N-H bond of an
Q35: Why are 1°,2°,and 3° alkylamines more basic
Q37: What is the name given to naturally
Q38: What is the major organic product obtained
Q39: Which of the following alkyl halides cannot
Q40: Rank the following compounds in order of
Q41: Select the reagent(s)required for the following transformation.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents