Which of the following letters represents DH° for the forward reaction in the following energy diagram? 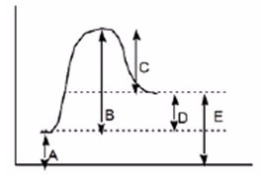
A) A
B) B
C) C
D) D
Correct Answer:
Verified
Q21: How many transition states and intermediates would
Q22: The DG° (free energy change)for the conversion
Q23: Which reaction is slowest? Q24: Which step would most likely have the Q25: Which of the following statements about a Q27: The equilibrium constant for the conversion of Q28: Which reaction has a positive DG°,assuming that Q29: Which of the following statements is not Q30: Which reaction is fast and has Keq Q31: Which of the following statements is true?![]()
A)The
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents