The conversion of acetyl chloride to methyl acetate occurs via the following two-step mechanism: 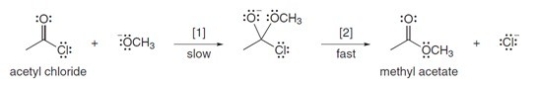 If the concentrations of both -OCH3 and acetyl chloride were increased 2 times,what would happen to the rate of the reaction?
If the concentrations of both -OCH3 and acetyl chloride were increased 2 times,what would happen to the rate of the reaction?
A) Rate would become one-fourth
B) Rate would increase 4 times
C) Rate would increase 16 times
D) Rate would increase 2 times
Correct Answer:
Verified
Q43: What type of bond cleavage takes place
Q44: For which of the following reactions is
Q45: The conversion of acetyl chloride to methyl
Q46: Which compound would you predict to be
Q47: The symbol hν stands for _ in
Q48: The conversion of acetyl chloride to methyl
Q50: What type of reaction does the following
Q51: Which of the following statements about a
Q52: The symbol Δ stands for _ in
Q53: The conversion of acetyl chloride to methyl
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents