An unknown compound X has the empirical formula C3H6O and a molecular ion in its mass spectrum at 116.Compound X shows no IR absorption at 3200-3600 cm-1 but shows a peak at 1700 cm-1.The 1H NMR spectral data of X is shown below.What is the structure of compound X? 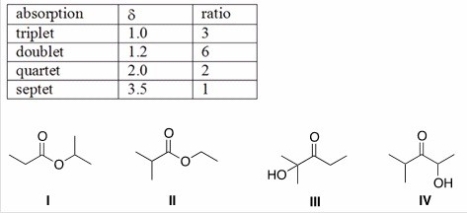
A) I
B) II
C) III
D) IV
Correct Answer:
Verified
Q35: Which of the indicated protons absorbs furthest
Q36: An unknown compound A has the molecular
Q37: An unknown compound A has the molecular
Q38: What is the relationship between Ha and
Q39: An unknown compound X has the molecular
Q42: How many different kinds of 13C peaks
Q44: How many different kinds of protons are
Q45: How many different kinds of 13C peaks
Q48: What effect does increasing the operating frequency
Q49: What effect does increasing the operating frequency
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents