Why can't you prepare 2-tert-butylcyclohexanone by the following reaction? 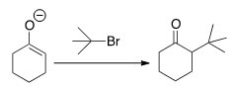
A) Because tert-butyl bromide is too basic.
B) Because tert-butyl bromide cannot undergo an SN2 reaction.
C) Because tert-butyl bromide is a nucleophile.
D) Because tert-butyl bromide is not a stable compound.
Correct Answer:
Verified
Q1: Which of the following is the intermediate
Q5: Starting with cyclohexanone,how could you prepare the
Q5: Why is the enolate of acetone less
Q7: Will acetone be completely deprotonated by potassium
Q8: Which of the following four compounds is
Q8: If you want to form a kinetic
Q12: If you want to form a thermodynamic
Q12: What is (are)the product(s)of the following reaction?
Q15: Why is it difficult to stop the
Q15: What is the missing reagent in the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents