The reaction below is a direct enolate alkylation.It has been found that this reaction only works well with unhindered methyl and 1° alkyl halides.Pick the statement that best explains this observation. 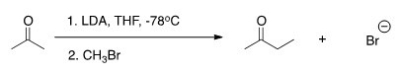
A) The nucleophilic enolate requires a reaction center that has a positive charge.
B) Hindered alkyl halides do not undergo SN1 reactions.
C) Hindered alkyl halides do not undergo SN2 reactions.
D) Methyl and 1° alkyl halides can form carbocations that can readily react with the nucleophilic enolate.
Correct Answer:
Verified
Q24: The malonic ester synthesis can be adapted
Q24: Which of the following bases will completely
Q25: Select the appropriate sequence of reactions to
Q26: A simple chemical test to distinguish between
Q27: What is the starting material in the
Q28: Which is the thermodynamic enolate of 2-methylcyclohexanone?
Q30: Treatment of 2-hexanone with NaOCH2CH3 followed by
Q31: Which is the most acidic proton in
Q32: Which of the following ketones will give
Q34: Which of the following compounds would undergo
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents