How would the following compounds be distinguishable using IR and 1H NMR spectroscopy? 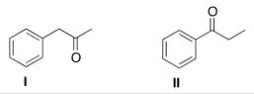
A) The 1H NMR spectrum of compound I will have two singlets.
B) The C=O absorption in the IR spectrum of compound I will be at a higher wave number than that of compound II.
C) The 1H NMR spectrum of compound II will have one triplet at a chemical shift of about 4.
D) The 1H NMR spectrum of compound I will have two singlets AND the C=O absorption in the IR spectrum of compound I will be at a higher wave number than that of compound II.
Correct Answer:
Verified
Q29: What is (are)the product(s)of the following reaction?
Q30: What is the product of the following
Q31: What is the missing reagent in the
Q32: What is the product of the following
Q33: What is the product of the following
Q35: What is the product of the following
Q36: What sequence of reactions is required for
Q37: Name the following aldehyde. Q38: What is (are)the product(s)of the following reaction? Q39: What needs to be done to make![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents