Which step would most likely have the largest energy of activation? 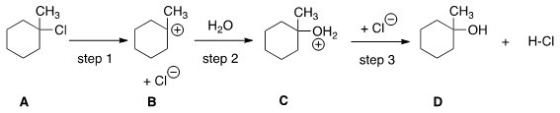
A) Step one
B) Step two
C) Step three
D) It cannot be determined from the information provided
Correct Answer:
Verified
Q26: In which reaction is Keq > 1?
Q27: Which reaction has a positive DG°,assuming that
Q29: Which of the following statements is not
Q30: Which reaction is slowest? Q31: Which of the following statements is true? Q32: Which of the following reaction quantities will Q32: What kind of reaction does the conversion Q33: Which reaction is fast and has Keq Q34: If the conversion of A to B Q36: The DG° (free energy change)for the conversion![]()
A)The
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents