Which of the following compounds would be expected to be more soluble in hexane (C6H14) ? 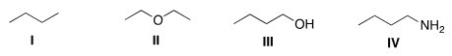
A) I
B) II
C) III
D) IV
Correct Answer:
Verified
Q27: What intermolecular force is generally considered the
Q35: Which of the following intermolecular forces would
Q36: Which of the following compounds is expected
Q37: Rank the following compounds in order of
Q38: Which of the following compounds has the
Q41: List the intermolecular forces present in the
Q42: Rank the following compounds in order of
Q43: Which of the following molecules can bond
Q44: List the intermolecular forces present in the
Q45: The indicated bond is: ![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents