Consider compounds which contain both a heteroatom and a double bond.For which compound is no additional Lewis structure possible? 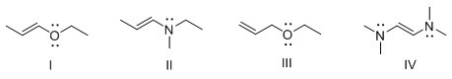
A) I
B) II
C) III
D) IV
Correct Answer:
Verified
Q47: What is the molecular geometry around the
Q48: What is the molecular geometry around the
Q57: Which of the following is the appropriate
Q58: What is the hybridization for each of
Q59: Determine the geometry around the indicated atom
Q62: Rank the following atoms in order of
Q63: When forming molecular orbitals from atomic orbitals,what
Q64: Which of the following molecules does not
Q66: Rank the following atoms in order of
Q71: Which atomic orbitals overlap to form the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents