Sodium chlorate is used as an oxidizer in the manufacture of dyes, explosives, and matches. Calculate the mass of solute needed to prepare 1.575 L of 0.00250 M NaClO3 ( 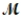 = 106.45 g/mol) .
= 106.45 g/mol) .
A) 419 g
B) 169 g
C) 0.419 g
D) 0.169 g
E) 0.00394 g
Correct Answer:
Verified
Q6: Which of the following is most soluble
Q7: Which of the following will be least
Q9: Sodium hydroxide, also known as caustic soda,
Q10: How many moles of H+(aq) ions are
Q12: Calculate the molarity of a 23.55-mL solution
Q23: How many moles of ions are released
Q26: What will be the final volume of
Q28: When 2.61 g of solid Na2CO3 is
Q29: What volume, in L, of 10.0 M
Q31: How many moles of H+(aq) ions are
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents