Magnesium (used in the manufacture of light alloys) reacts with iron(III) chloride to form magnesium chloride and iron. 3Mg(s) + 2FeCl3(s) → 3MgCl2(s) + 2Fe(s) A mixture of 41.0 g of magnesium ( 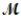 = 24.31 g/mol) and 175 g of iron(III) chloride (
= 24.31 g/mol) and 175 g of iron(III) chloride ( 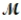 = 162.2 g/mol) is allowed to react. Identify the limiting reactant and determine the mass of the excess reactant present in the vessel when the reaction is complete.
= 162.2 g/mol) is allowed to react. Identify the limiting reactant and determine the mass of the excess reactant present in the vessel when the reaction is complete.
A) Limiting reactant is Mg; 67 g of FeCl3 remain.
B) Limiting reactant is Mg; 134 g of FeCl3 remain.
C) Limiting reactant is Mg; 104 g of FeCl3 remain.
D) Limiting reactant is FeCl3; 2 g of Mg remain.
E) Limiting reactant is FeCl3; 87 g of Mg remain.
Correct Answer:
Verified
Q25: Determine the percent composition of potassium dichromate,
Q39: Alkanes are compounds of carbon and hydrogen
Q41: Aluminum oxide (used as an adsorbent or
Q42: Magnesium reacts with iron(III) chloride to form
Q42: Balance the following equation: B2O3(s) + HF(l)
Q44: Balance the following equation: Ca3(PO4)2(s) + SiO2(s)
Q49: How many molecules of molecular oxygen react
Q50: Aluminum will react with bromine to form
Q51: Lead(II) sulfide was once used in glazing
Q69: Potassium chloride is used as a substitute
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents