Identify the missing species in the following nuclear transmutation. 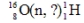
A) 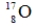
B) 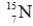
C) 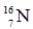
D) 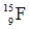
E) 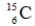
Correct Answer:
Verified
Q45: The isotope Q45: Iodine-131, t1/2 = 8.0 days, is used Q46: A 55-kg person exposed to thorium-234 receives Q47: A 30.0-kg child receives 2.65 × 107 Q49: An 85-kg person exposed to barium-141 receives Q51: Identify the missing species in the following Q53: Identify the missing species in the following Q57: Cesium-134 is a β emitter with a Q59: Palladium-107 undergoes β decay (t1/2 = 6.5 Q77: In living organisms, C-14 atoms disintegrate at![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents