Which of the following conditions is most likely to apply to a fully-charged secondary cell?
A) E cell l = E° cell
B) E° cell = 0
C) 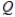 = 1
= 1
D) 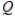 < K
< K
E) 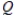 = K
= K
Correct Answer:
Verified
Q20: Which of the following solids is commonly
Q21: The redox reaction of peroxydisulfate with iodide
Q22: Examine the following half-reactions and select the
Q26: When metal A is placed in a
Q27: Examine the following half-reactions and select the
Q28: Calculate E°cell and indicate whether the overall
Q29: What is the E°cell for the cell
Q30: A voltaic cell can be prepared from
Q45: The voltaic cell made up of cobalt,
Q60: A voltaic cell has a standard cell
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents