Multiple Choice
Iron(III) oxide can be reduced by carbon monoxide. Fe2O3(s) + 3CO(g)  2Fe(s) + 3CO2(g)
2Fe(s) + 3CO2(g)
Use the following thermodynamic data at 298 K to determine the equilibrium constant at this temperature.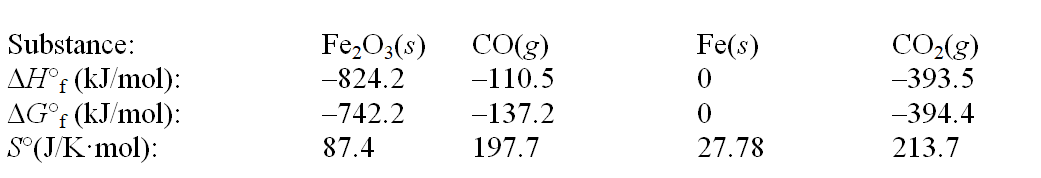
A) 7.0 × 10-6
B) 1.3 × 10-3
C) 2.2 × 104
D) 1.4 × 105
E) > 2.0 × 105
Correct Answer:
Verified
Related Questions
Q4: As a chemical reaction proceeds toward equilibrium,
Q9: For any reaction, if ΔG° > 0,
Q10: The higher the pressure of a gas
Q14: For a reaction at equilibrium, ΔSuniv =
Q76: A reaction has ΔG = 10.0 kJ
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents