Calculate the equilibrium constant at 25°C for the reaction of methane with water to form carbon dioxide and hydrogen. The data refer to 25°C. CH4(g) + 2H2O(g)  CO2(g) + 4H2(g)
CO2(g) + 4H2(g) 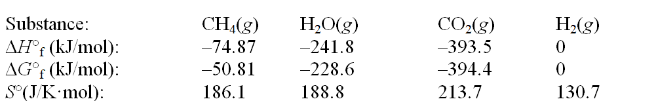
A) 8.2 × 1019
B) 0.96
C) 0.58
D) 1.2 × 10-20
E) 1.4 × 10-46
Correct Answer:
Verified
Q6: Under a given set of conditions, all
Q7: The free energy of a perfect crystal
Q12: The term microstate refers to the energy
Q16: The entropy of one mole of oxygen
Q17: For a given reaction, a change in
Q59: Calculate ΔG° for the reaction SiCl4(g) +
Q60: Given: H2O(l) → H2O(g) ΔH° = 40.7
Q61: The formation constant for the reaction Ag+(aq)
Q62: Consider the figure that shows ΔG° for
Q65: What is the free energy change, ΔG°,
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents