The reaction of methane with water to form carbon dioxide and hydrogen is nonspontaneous at 298 K. At what temperature will this system make the transition from nonspontaneous to spontaneous? The data refer to 298 K. CH4(g) + 2H2O(g)  CO2(g) + 4H2(g)
CO2(g) + 4H2(g) 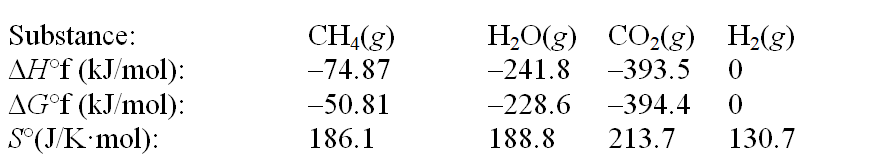
A) 658 K
B) 683 K
C) 955 K
D) 1047 K
E) 1229 K
Correct Answer:
Verified
Q6: Under a given set of conditions, all
Q10: The higher the pressure of a gas
Q11: In some spontaneous processes, the entropy of
Q12: The term microstate refers to the energy
Q13: In a spontaneous process, the entropy of
Q16: The entropy of one mole of oxygen
Q17: For a given reaction, a change in
Q65: What is the free energy change, ΔG°,
Q71: Use the thermodynamic data at 298 K
Q80: A reaction is proceeding toward equilibrium. At
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents