Write the mass-action expression,  c, for the following chemical reaction. Sn2+(aq) + ½O2(g) + 3H2O(l)
c, for the following chemical reaction. Sn2+(aq) + ½O2(g) + 3H2O(l)  SnO2(s) + 2H3O+(aq)
SnO2(s) + 2H3O+(aq)
A) 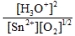
B) 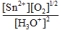
C) 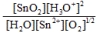
D) 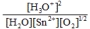
E) None of these choices are correct.
Correct Answer:
Verified
Q17: Carbon monoxide and chlorine combine in an
Q18: Write the mass-action expression, Q19: Consider the equilibrium reaction shown below. B2(g) Q20: Write the mass-action expression, Q21: Consider the following two equilibria and their Q23: The equilibrium constant for reaction (1) below Q24: The equilibrium constant, Kc, for the decomposition Q25: The equilibrium constant, Kp, for the reaction Q26: What is the mass-action expression, Q27: The reaction of nitrogen with oxygen to![]()
![]()

Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents