Nitric oxide and bromine were allowed to react in a sealed container. When equilibrium was reached PNO = 0.526 atm, 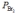 = 1.59 atm, and PNOBr = 7.68 atm. Calculate Kp for the reaction.
= 1.59 atm, and PNOBr = 7.68 atm. Calculate Kp for the reaction.
2NO(g) + Br2(g)  2NOBr(g)
2NOBr(g)
A) 7.45 × 10-3
B) 0.109
C) 9.18
D) 91.8
E) 134
Correct Answer:
Verified
Q37: Hydrogen sulfide will react with water as
Q38: Consider the equilibrium reaction: N2O4(g) 
Q39: Consider the reactions of cadmium with the
Q40: At 500°C the equilibrium constant, Kp, is
Q41: 10.0 mL of a 0.100 mol L-1
Q44: At 850°C, the equilibrium constant Kp for
Q45: At 25°C, the equilibrium constant Kc for
Q47: Ammonium iodide dissociates reversibly to ammonia and
Q63: SO2 reacts with O2 to produce SO3.
Q65: Hydrogen iodide, HI, is formed in an
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents