Saccharin, one of the first non-nutritive sweeteners used in soft-drinks, is 500 times sweeter than sugar in dilute aqueous solutions. The solubility of saccharin is 1.00 gram per 290 mL of solution. What is the molarity of a saturated saccharin solution? ( 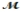 saccharin = 183.2 g/mol)
saccharin = 183.2 g/mol)
A) 0.0188 M
B) 0.632 M
C) 1.58 M
D) 3.45 M
E) None of these choices are correct.
Correct Answer:
Verified
Q22: Calculate the molarity of a solution prepared
Q37: What volume of concentrated (14.7 M) phosphoric
Q43: The concentration of iodine in sea water
Q48: The chemist, Anna Lytic, must prepare 1.00
Q49: Aqueous ammonia is commercially available in a
Q52: Colligative properties depend on
A)the chemical properties of
Q57: What is the mole fraction of Ne
Q58: A 2.0% (w/v) solution of sodium hydrogen
Q72: Which of the following aqueous solutions will
Q77: What concentration of aqueous FeCl3 would have
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents