Isoamyl salicylate ( 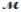 = 208.25 g/mol) has a pleasant aroma and is used in perfumes and soaps. Which of the following combinations gives a 0.75 m solution of isoamyl salicylate in ethyl alcohol (d = 0.7893 g/mL) ?
= 208.25 g/mol) has a pleasant aroma and is used in perfumes and soaps. Which of the following combinations gives a 0.75 m solution of isoamyl salicylate in ethyl alcohol (d = 0.7893 g/mL) ?
A) 117.2 g isoamyl salicylate in 950.0 mL of ethyl alcohol
B) 117.2 g isoamyl salicylate in 750.0 mL of ethyl alcohol
C) 117.2 g isoamyl salicylate in 750.0 mL of solution
D) 117.2 g isoamyl salicylate in 592.0 g of ethyl alcohol
E) None of these choices are correct.
Correct Answer:
Verified
Q22: Calculate the molarity of a solution prepared
Q37: What volume of concentrated (14.7 M) phosphoric
Q43: What is the molality of a solution
Q45: Sodium hydroxide is a common ingredient in
Q46: A solution of ethanol (C2H6O) in water
Q49: Aqueous ammonia is commercially available in a
Q51: A solution of ethanol (C2H6O) in water
Q54: The solubility of the oxidizing agent potassium
Q57: What is the mole fraction of Ne
Q58: A 2.0% (w/v) solution of sodium hydrogen
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents