In the valence bond treatment, a 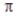 bond is formed when two 2p orbitals overlap side to side.
bond is formed when two 2p orbitals overlap side to side.
Correct Answer:
Verified
Q4: In applying molecular orbital theory, the bond
Q10: Valence bond theory is able to explain
Q14: In molecular orbital theory, combination of two
Q15: Valence bond theory explains the bonding in
Q37: How many sigma and pi bonds, respectively,
Q41: According to the molecular orbital (MO) treatment
Q42: In molecular orbital theory, combination of two
Q43: The lone pair of electrons on sulfur
Q44: According to molecular orbital (MO) theory, the
Q48: One can safely assume that the 3s-
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents