Calculate the mass defect for an atom of 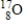 whose isotope mass is 16.9991 g/mol. mass of proton (
whose isotope mass is 16.9991 g/mol. mass of proton ( 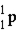 )
)
1) 00783 g/mol
Mass of neutron ( 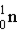 )
)
1) 00867 g/mol
A) 0.153 g/mol
B) 0.152 g/mol
C) 0.148 g/mol
D) 0.447 g/mol
E) 0.142 g/mol
Correct Answer:
Verified
Q17: If a nucleus gains a neutron and
Q18: What is the charge of a positron?
A)
Q19: Arrange the following types of radiation from
Q20: If astatine-207 decays by two α emission
Q21: Radioactive isotopes that sit above the band
Q23: Which of the following is the most
Q24: The half-life for the spontaneous decay of
Q25: Which of the following processes is responsible
Q26: Which of the following nuclides has the
Q27: The half-life of 42K is 12.5 h.How
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents