The number of protons in 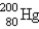 is _____.
is _____.
A) 80
B) 119
C) 199
D) dependent on ionic charge
E) unknown
Correct Answer:
Verified
Q144: Which of these are isotopes of hydrogen?
A)
Q145: The number of protons in the nucleus
Q146: 15N and 15O are isotopes.
Q147: Determine the number of protons and the
Q148: Give the name and symbol for the
Q150: How many neutrons are contained in an
Q151: An atom that has 54 protons,54 electrons,and
Q152: The number of neutrons in 
Q153: How many electrons are present in a
Q154: Beyond _,the neutron-to-proton ratio is always greater
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents