Metal hydrides are used to remove traces of water from nonpolar solvents.Write a balanced chemical equation for the reaction of lithium hydride and water.
A) LiH2(s) + 2 H2O( 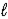 ) → Li(s) + 2 H3O+(aq)
) → Li(s) + 2 H3O+(aq)
B) 2 LiH(s) + 2 H2O( 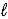 ) → 2 LiOH(s) + H2(g)
) → 2 LiOH(s) + H2(g)
C) 2 LiH(s) + H2O( 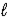 ) → Li2O(s) + 2 H2(g)
) → Li2O(s) + 2 H2(g)
D) 2 LiH(s) + H2O( 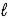 ) → Li2O(s) + 4 H+(aq)
) → Li2O(s) + 4 H+(aq)
E) LiH(s) + H2O( 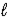 ) → Li(s) + H3O+(aq)
) → Li(s) + H3O+(aq)
Correct Answer:
Verified
Q3: What is the oxidation state of the
Q4: What is the formula of the common
Q5: Approximately 300 billion liters of hydrogen is
Q6: What is the highest oxidation state possible
Q7: Which of the following is the second
Q9: Use your knowledge of group properties to
Q10: Which of the following reactions does not
Q11: The oxidation state per each nitrogen,given in
Q12: Which of the following reactions describes the
Q13: Which of the following is the most
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents