Write a balanced chemical equation for the electrolysis of brine (salt water) .
A) 2 NaCl(aq) + 2 H2O( 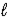 ) → 2 Na(s) + 2 HCl(aq) + O2(g)
) → 2 Na(s) + 2 HCl(aq) + O2(g)
B) 2 NaCl( 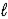 ) → 2 Na(
) → 2 Na( 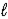 ) + Cl2(g)
) + Cl2(g)
C) 2 NaCl(aq) + O2(g) → Na2O2(s) + Cl2(g)
D) 4 NaCl(aq) + O2(g) → 4 Na(s) + 2 OCl2(g)
E) 2 NaCl(aq) + 2 H2O( 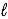 ) → Cl2(g) + 2 NaOH(aq) + H2(g)
) → Cl2(g) + 2 NaOH(aq) + H2(g)
Correct Answer:
Verified
Q24: Which of the following calcium compounds is
Q25: Which of the following methods is used
Q26: What mass of MgO would be produced
Q27: Which one of the following elements would
Q28: Which of the following is the general
Q30: The primary source material for the commercial
Q31: Which of the following elements does not
Q32: Which of the following statements is INCORRECT?
A)
Q33: Write a balanced chemical equation for the
Q34: Oxides of the alkaline earth family form
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents