Write a balanced half-reaction for the reduction of CrO42-(aq) to Cr(OH) 3(s) in a basic solution.
A) CrO42-(aq) + 3 OH-(aq) + 3 e- → Cr(OH) 3(s) + 2 O2(g)
B) CrO42-(aq) + 3 H+(aq) + 3 e- → Cr(OH) 3(s)
C) CrO42-(aq) + 3 H+(aq) → Cr(OH) 3(s) + 2 e-
D) CrO42-(aq) + 4 H2O( 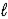 ) + 3 e- → Cr(OH) 3(s) + 5 OH-(aq)
) + 3 e- → Cr(OH) 3(s) + 5 OH-(aq)
E) CrO42-(aq) + 3 OH-(aq) → Cr(OH) 3(s) + 2 O2(g)
Correct Answer:
Verified
Q7: Balance the following oxidation-reduction reaction occurring in
Q8: Balance the following half-reaction occurring in a
Q9: Write a balanced net ionic equation for
Q10: Write a balanced half-reaction for the reduction
Q11: Which of the following reactions will require
Q13: Which of the following statements is true
Q14: Which of the following statements is true
Q15: How many electrons are transferred in the
Q16: When the given oxidation-reduction reaction in an
Q17: Write the balanced reduction half-reaction for the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents