Calculate 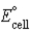 for the electrochemical cell Pb(s) |PbCl2(s) | Cl-(aq,1.0 M) || Fe3+(aq,1.0 M) ,Fe2+(aq,1.0 M) | Pt(s) . The standard reduction potentials are as follows:
for the electrochemical cell Pb(s) |PbCl2(s) | Cl-(aq,1.0 M) || Fe3+(aq,1.0 M) ,Fe2+(aq,1.0 M) | Pt(s) . The standard reduction potentials are as follows:
Pb2+(aq) + 2 e- → Pb(s)
E° = -0.126 V
PbCl2(s) + 2 e- → Pb(s) + 2 Cl-(aq)
E° = -0.267 V
Fe3+(aq) + e- → Fe2+(aq)
E° = +0.771 V
Fe2+(aq) + e- → Fe(s)
E° = -0.44 V
A) -0.504 V
B) -0.062 V
C) +0.504 V
D) +1.038 V
E) +1.604 V
Correct Answer:
Verified
Q49: Calculate the value of the reaction quotient,Q,for
Q50: Calculate Ecell for the following electrochemical cell
Q51: The following has a potential of 0.34
Q52: Calculate the cell potential at 25 °C
Q53: For the following cell reaction,the standard cell
Q55: Calculate Ecell for the following electrochemical cell
Q56: Calculate Ecell for the following electrochemical cell
Q57: For the electrochemical cell Cu(s)| Cu2+ ||
Q58: Which of the following is true for
Q59: One Faraday is defined as the:
A) charge
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents