The following has a potential of 0.34 V. 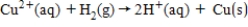 If the concentrations of each of the ions is 1.0 M and the pressure of H2 is 1.0 atm,then E° for the half-reaction
If the concentrations of each of the ions is 1.0 M and the pressure of H2 is 1.0 atm,then E° for the half-reaction 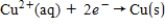 is _____.
is _____.
A) 0.17 V
B) -0.17 V
C) 0.34 V
D) -0.34 V
E) None of these
Correct Answer:
Verified
Q46: If the Q47: The standard cell potential of the given Q48: The cell potential of the following electrochemical Q49: Calculate the value of the reaction quotient,Q,for Q50: Calculate Ecell for the following electrochemical cell Q52: Calculate the cell potential at 25 °C Q53: For the following cell reaction,the standard cell![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents