Which of the following equations represents the Nernst equation?
A) 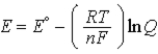
B) 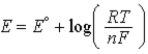
C) 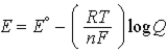
D) 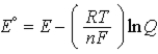
E) 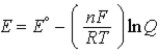
Correct Answer:
Verified
Q37: Which of the following is the cell
Q38: Write a balanced net ionic equation for
Q39: Which of the following is the cell
Q40: Which of the following overall chemical equations
Q41: The standard reduction potentials are as follows:
Q43: Which of the following statements is true
Q44: The following electrochemical cell has a potential
Q45: Calculate the copper(II)ion concentration at 25 °C
Q46: If the Q47: The standard cell potential of the given![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents