A voltaic cell or galvanic cell converts chemical energy to electrical energy. 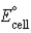 for the given galvanic cell is -1.80 V. Fe2+(aq) + 2 Cl-(aq) → Fe(s) + Cl2(g)
for the given galvanic cell is -1.80 V. Fe2+(aq) + 2 Cl-(aq) → Fe(s) + Cl2(g)
Calculate the value of ΔrG° for the reaction.
A) -174 kJ
B) 86.8 kJ
C) 107 kJ
D) 174 kJ
E) 347 kJ
Correct Answer:
Verified
Q72: The use of electrical energy to produce
Q73: Aluminum(III)ion (Al3+)is reduced to solid aluminum at
Q74: Calculate the value of the equilibrium constant
Q75: Calculate ΔrG° for the disproportionation reaction of
Q76: The standard reduction potentials for a reaction
Q78: Gold and platinum are commonly used as
Q79: Calculate the equilibrium constant for the following
Q80: How many moles of electrons are produced
Q81: Explain the function of a salt bridge
Q82: If an electric current is passed through
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents