The value of E°cell is 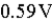 for the following reaction: Cl2(g) + 2 Fe2+(aq) → 2 Fe3+(aq) + 2 Cl-(aq)
for the following reaction: Cl2(g) + 2 Fe2+(aq) → 2 Fe3+(aq) + 2 Cl-(aq)
Calculate the value of E°cell for the reaction below.
Cl-(g) + Fe3+(aq) → Fe2+(aq) + ½ Cl2(g)
A) -1.18 V
B) -0.30 V
C) 0.59 V
D) 0.30 V
E) -0.59 V
Correct Answer:
Verified
Q58: Which of the following is true for
Q59: One Faraday is defined as the:
A) charge
Q60: Calculate Q61: Calculate the standard reduction potential for the Q62: Claculate the mass of chromium that can Q64: What half-reaction occurs at the cathode during Q65: In an electrolytic cell,reduction occurs at the Q66: Which of the following are the expected Q67: If ΔrG° for the following reaction is Q68: Calculate the equilibrium constant for the reaction![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents