Hydrogen gas is prepared by electrolysis of water according to the reaction below. 2 H2O( 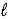 ) → 2 H2(g) + O2(g)
) → 2 H2(g) + O2(g)
Predict the signs of ΔrH and ΔrS.
A) ΔrH > 0 and ΔrS > 0
B) ΔrH < 0 and ΔrS > 0
C) ΔrH > 0 and ΔrS < 0
D) ΔrH < 0 and ΔrS < 0
E) ΔrH = 0 and ΔrS < 0
Correct Answer:
Verified
Q30: Calculate ΔrG° at 25.0 °C for the
Q31: ΔG° = 0 for a reaction indicates
Q32: A flask containing helium gas is released
Q33: If a cube of ice at 0
Q34: For a reaction,ΔrH° = -208.8 kJ and
Q36: For a chemical reaction,if ΔrG° = 0,then
Q37: Which of the following is correct for
Q38: Which of the following is true of
Q39: When a real gas is compressed from
Q40: The dissolution of ammonium nitrate occurs spontaneously
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents